Quality Management System: A Guide to Effective Implementation
A Quality Management System is not just a set of procedures; it’s a comprehensive framework that aligns an organization’s processes, people, and resources towards the common goal of delivering consistent quality.
Quality Management System (QMS) can drive continuous improvement, mitigate risks, and create a culture of quality that permeates every level of an organization.
Key Highlights
- Definition and evolution of Quality Management Systems
- Essential components of an effective QMS
- Role of Six Sigma certification in building quality management expertise
- Step-by-step guide to implementing a QMS, from planning to internal audits
- Continuous improvement methodologies
- Overview of QMS standards, with a focus on ISO 9001 certification
- Practical applications of QMS in enhancing customer satisfaction and supplier management
- Future trends in QMS
What is Quality Management Systems?
A Quality Management System (QMS) is a structured framework of processes, procedures, and responsibilities designed to ensure consistent quality in an organization’s products or services.
A well-designed QMS serves as the backbone of an organization’s quality assurance efforts.
It provides a systematic approach to managing quality-related activities, from product design to customer service.
The ultimate goal is to enhance customer satisfaction, improve operational efficiency, and foster a culture of continuous improvement.
Evolution of Quality Management
The concept of quality management has come a long way since the early days of industrial manufacturing.
The journey began with basic quality control measures in the early 20th century, focusing primarily on product inspection.
As industries grew more complex, statistical process control techniques emerged, allowing for more proactive quality management.
The post-World War II era saw the rise of Total Quality Management (TQM), emphasizing company-wide quality efforts.
Today’s Quality Management System (QMS) integrates advanced methodologies like Six Sigma and Lean manufacturing, which I’ve had the privilege of implementing in various organizations.
These modern approaches focus on data-driven decision-making and waste reduction, pushing the boundaries of what’s possible in quality management.
Key Components of an Effective Quality Management System
At the heart of any effective Quality Management System (QMS) lies a clear and concise quality policy.
This high-level document outlines an organization’s commitment to quality and sets the tone for all quality-related activities.
Complementing the quality policy are specific, measurable quality objectives.
These objectives should be aligned with the overall business strategy and cascaded throughout the organization.
For instance, when I worked with Dell, we established objectives around reducing defect rates and improving customer satisfaction scores, which were then translated into department-specific goals.
Document Control and Quality Manual
Effective document control is crucial for maintaining the integrity of a Quality Management System (QMS).
This involves managing the creation, approval, distribution, and revision of all quality-related documents.
A well-structured document control system ensures that everyone in the organization has access to the most up-to-date information and procedures.
The quality manual serves as the cornerstone document of the QMS, providing an overview of the entire system.
It typically includes the quality policy, objectives, and a description of how the organization meets the requirements of the chosen quality standard (e.g., ISO 9001).
In my consulting work, I often emphasize the importance of creating a concise, user-friendly quality manual that serves as a roadmap for the entire Quality Management System.
Process Approach to Quality Management
A process approach to quality management is fundamental to modern QMS implementations.
This approach views the organization as a system of interrelated processes, rather than isolated departments or functions.
By mapping and optimizing these processes, organizations can improve efficiency and reduce variability.
Risk Management in Quality Management System
Integrating risk management into the Quality Management System is a relatively recent development, but one that I’ve found to be incredibly valuable.
By identifying potential risks to quality and implementing preventive measures, organizations can proactively address issues before they impact customers or regulatory compliance.
For example, in pharmaceutical companies, tools like Failure Mode and Effects Analysis (FMEA) are used to identify and mitigate potential quality risks in drug manufacturing processes.
This risk-based thinking helps organizations allocate resources more effectively and improve overall system resilience.
Implementing a Quality Management System
Implementing a Quality Management System requires careful planning and a systematic approach.
The first step is to conduct a gap analysis to identify where current practices fall short of the desired quality standard.
Based on this analysis, a detailed implementation plan can be developed.
When I led the Six Sigma deployment at 3M, we started by mapping out key processes and identifying areas for improvement.
This involved engaging stakeholders from across the organization to ensure buy-in and alignment with business objectives.
The plan should include timelines, resource allocation, and clear responsibilities for each aspect of the QMS implementation.
Developing Standard Operating Procedures
Standard Operating Procedures (SOPs) are the backbone of a consistent and effective Quality Management System.
These detailed, step-by-step instructions ensure that critical processes are performed consistently, regardless of who is performing them.
When developing SOPs, it’s crucial to involve the people who actually perform the work. Their insights are invaluable in creating procedures that are both practical and effective.
Training Management for Quality Management System Success
A Quality Management System is only as effective as the people implementing it. That’s why comprehensive training is crucial for QMS success.
Training should cover not just the specific procedures and tools of the Quality Management System, but also the underlying principles of quality management.
Internal Audits and Management Review
Regular internal audits are essential for maintaining the effectiveness of a QMS.
These audits help identify areas of non-conformance, opportunities for improvement, and best practices that can be shared across the organization.
Management review is another critical component of Quality Management System implementation.
This regular review process ensures that top management stays engaged with the QMS and that the system remains aligned with the organization’s strategic objectives.
Continuous Improvement in Quality Management Systems
PDCA Cycle for Ongoing Enhancement
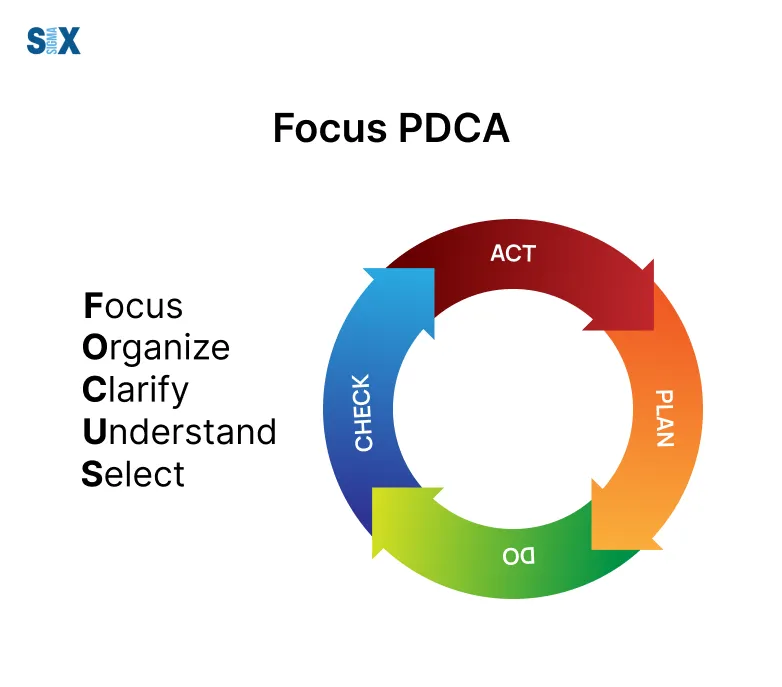
The Plan-Do-Check-Act (PDCA) cycle is a fundamental tool for driving continuous improvement in a Quality Management System.
This iterative four-step approach provides a structured method for problem-solving and process improvement.
In the ‘Plan‘ phase, we identify opportunities for improvement and develop action plans. The ‘Do‘ phase involves implementing these plans on a small scale.
In the ‘Check‘ phase, we analyze the results to see if the desired improvements were achieved.
Finally, in the ‘Act‘ phase, we either standardize the successful changes or begin the cycle again with modified plans.
Corrective and Preventive Actions
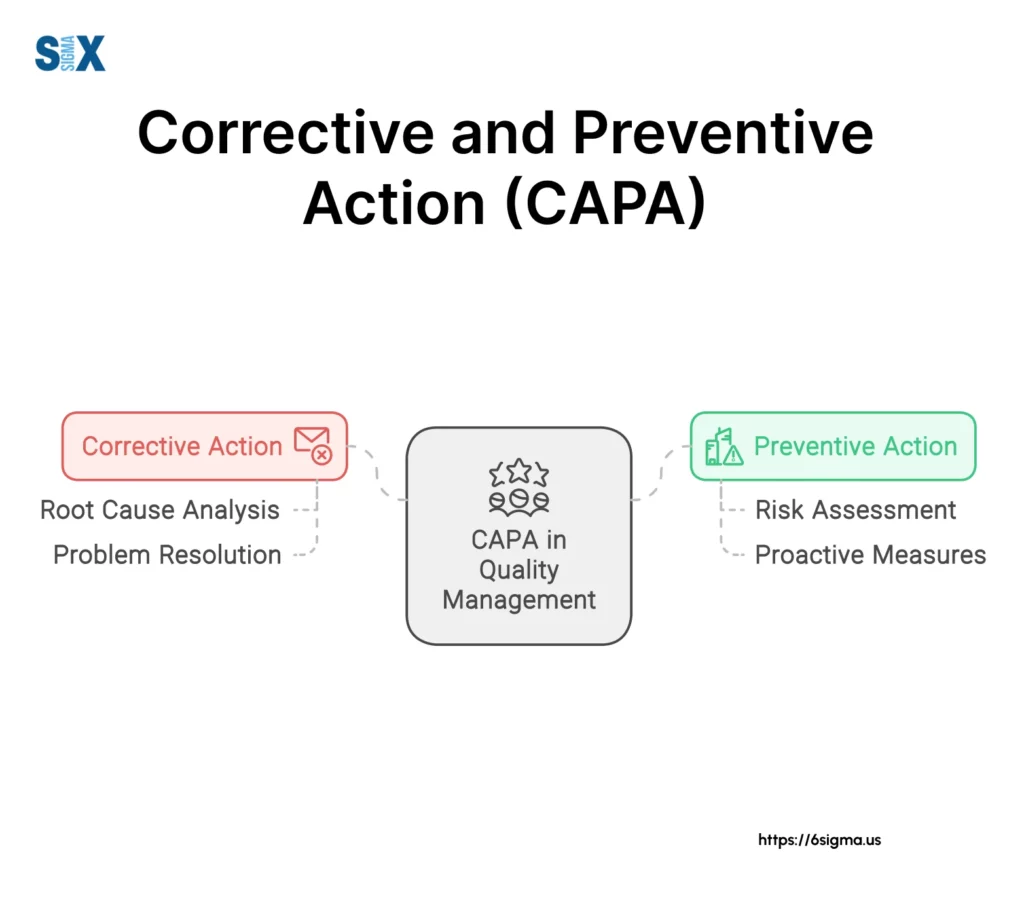
Corrective and Preventive Actions (CAPA) are crucial elements of a Quality Management System that help organizations address existing problems and prevent potential issues.
Corrective actions deal with non-conformities that have already occurred, while preventive actions aim to eliminate the causes of potential non-conformities.
A well-managed CAPA process can turn quality issues into opportunities for significant process improvements.
Performance Metrics and Quality Tools
Effective performance measurement is essential for driving continuous improvement in a Quality Management System (QMS).
Key Performance Indicators (KPIs) should be carefully selected to provide meaningful insights into the quality of processes, products, and services.
There’s a wealth of quality tools available to support continuous improvement efforts.
These tools provide powerful ways to analyze data, identify improvement opportunities, and optimize processes.
Root Cause Analysis Techniques
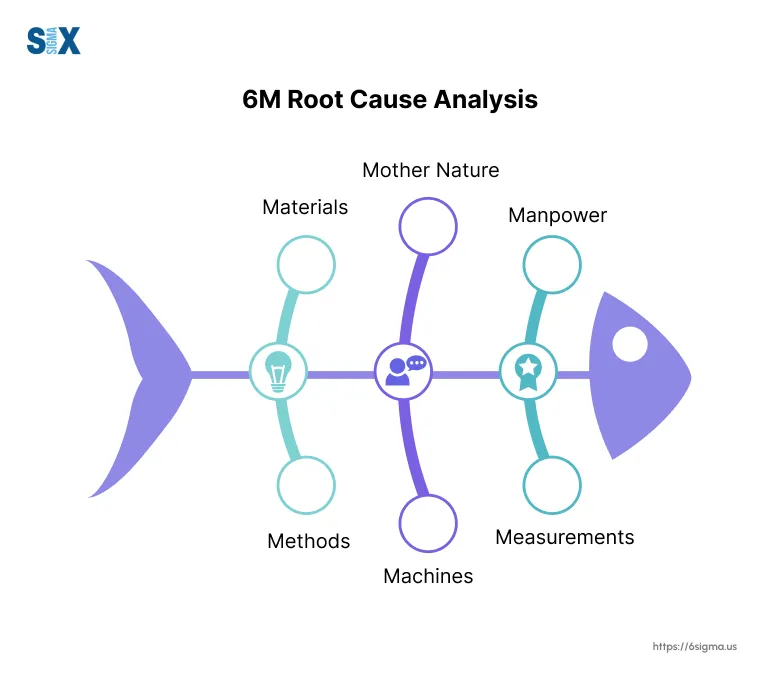
Root Cause Analysis (RCA) is a critical skill in quality management, allowing organizations to address the underlying causes of problems rather than just treating symptoms.
Techniques like the ‘5 Whys‘ and Fishbone Diagrams covered in root cause analysis training can be incredibly effective in uncovering root causes.
This balanced approach helps ensure that the true root causes are identified and addressed, leading to more effective and lasting improvements.
Quality Management System Standards and Certifications
Overview of ISO 9001 Requirements
ISO 9001 is the most widely recognized international standard for quality management systems.
It provides a set of requirements that organizations can use to develop and implement an effective Quality Management System.
The standard is based on seven quality management principles: customer focus, leadership, engagement of people, process approach, improvement, evidence-based decision making, and relationship management.
Certification Process and Benefits
The ISO 9001 certification process typically involves several stages, including gap analysis, documentation development, implementation, internal audits, and finally, the certification audit by an accredited certification body.
Certification to ISO 9001 can lead to improved operational efficiency, enhanced customer satisfaction, and increased market opportunities.
It provides a framework for consistent quality management practices and demonstrates a commitment to quality to customers and stakeholders.
However, it’s important to remember that certification is not the end goal – the real value comes from the ongoing implementation and improvement of the QMS.
Other Quality Management System (QMS) Frameworks: Six Sigma and Lean
While ISO 9001 provides a solid foundation for quality management, many organizations choose to integrate other methodologies to enhance their Quality Management System.
Six Sigma, which I’ve extensively applied in my career, focuses on reducing process variation and defects. It uses a data-driven approach and statistical tools to drive improvements.
Lean manufacturing, on the other hand, focuses on eliminating waste and improving flow in processes.
I’ve found that combining Lean principles with Six Sigma (often referred to as Lean Six Sigma) can be particularly powerful, addressing both process efficiency and quality simultaneously.
These methodologies can be effectively integrated into an ISO 9001-based QMS, providing additional tools and techniques for continuous improvement and problem-solving.
Organizations often train teams in tiered programs, from Six Sigma Yellow Belt certification (basic principles) to Six Sigma Black Belt certification (advanced statistical analysis).
Quality Management System in Practice: Ensuring Customer Satisfaction and Compliance
QMS should be designed to meet and exceed customer requirements.
This involves not just understanding current customer needs, but also anticipating future requirements. In my work with companies across various industries, I’ve emphasized the importance of integrating Voice of the Customer (VOC) data into the Quality Management System.
Effective QMS practices for meeting customer requirements include regular customer surveys, complaint management systems, and mechanisms for incorporating customer feedback into product and process improvements.
For instance, when working with a major electronics manufacturer, we implemented a closed-loop system that ensured customer complaints were not just resolved, but also analyzed for potential systemic improvements.
Supplier Evaluation and Management
Supplier quality is a critical component of overall product or service quality. A robust Quality Management System should include processes for evaluating, selecting, and managing suppliers.
This typically involves supplier audits, performance metrics, and continuous improvement initiatives.
Nonconformity Management and Quality Control
Effective management of nonconformities is crucial for maintaining the integrity of a QMS.
This involves not just identifying and addressing nonconforming products or processes, but also analyzing trends to prevent recurrence.
Quality control measures, such as inspection plans and statistical process control, play a key role in detecting nonconformities before they reach the customer.
Future Trends in Quality Management Systems
The future of quality management systems is increasingly digital. Advanced technologies like artificial intelligence, machine learning, and the Internet of Things (IoT) are being integrated into Quality Management System.
QMS processes, offering new opportunities for real-time monitoring, predictive analytics, and automated decision-making.
IoT sensors can provide continuous, real-time data on process parameters, allowing for immediate detection of out-of-specification conditions.
Machine learning algorithms can analyze this data to predict potential quality issues before they occur, enabling proactive interventions.
AI-powered systems can automate routine quality checks and decision-making processes, freeing up human resources for more complex problem-solving tasks.
However, it’s crucial to remember that technology is a tool, not a solution in itself.
The successful integration of these technologies requires a solid foundation in quality management principles and a clear understanding of the organization’s quality objectives.
Risk-Based Thinking in Modern Quality Management System (QMS) Approaches
Risk-based thinking is becoming increasingly central to modern Quality Management System (QMS) approaches.
This involves systematically identifying, assessing, and prioritizing risks related to quality throughout the organization. The goal is to embed risk management into all quality-related decisions and processes.
The future of QMS will likely see an even greater emphasis on predictive risk management, leveraging big data and advanced analytics to identify potential quality risks before they materialize.
In conclusion, the field of quality management systems is dynamic and ever-evolving.
From the integration of cutting-edge technologies to the adoption of risk-based thinking and industry-specific adaptations, the future of Quality Management System (QMS) promises exciting opportunities for organizations to enhance their quality performance.
SixSigma.us offers both Live Virtual classes as well as Online Self-Paced training. Most option includes access to the same great Master Black Belt instructors that teach our World Class in-person sessions. Sign-up today!
Virtual Classroom Training Programs Self-Paced Online Training Programs






